

|
AM I DEPRESSED? TAKE A QUICK SURVEY TO FIND OUT Depression Survey |
|
IS TMS RIGHT FOR ME? TAKE A QUICK SURVEY TO FIND OUT TMS Survey |
Which patients are good candidates for NeuroStar TMS Therapy?
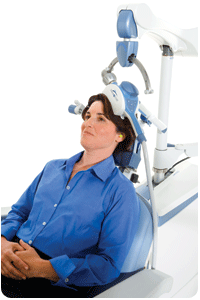 NeuroStar TMS Therapy is an appropriate treatment option for adult patients with Major Depressive Disorder who have failed to achieve satisfactory improvement from one prior antidepressant medication at or above the minimal effective dose and duration in the current episode.
NeuroStar TMS Therapy is an appropriate treatment option for adult patients with Major Depressive Disorder who have failed to achieve satisfactory improvement from one prior antidepressant medication at or above the minimal effective dose and duration in the current episode.
In clinical trials, half of the patients had been treated with at least four medication treatment attempts, one of which was at an adequate dose and duration.
NeuroStar TMS Therapy has not been studied in patients who have not received prior antidepressant treatment. Efficacy has not been established in patients who have failed to receive benefit from two or more prior antidepressant medications at minimal effective dose and duration in the current episode.
Which patients should not receive NeuroStar TMS Therapy?
NeuroStar TMS Therapy should not be used in patients with implanted metallic devices or non-removable metallic objects in or around the head (for example, metal plates in the skull, aneurysm coils, etc.) because serious injury could result.
Patients with braces and metal fillings are acceptable for treatment; however, patients with other metal within their mouth should discuss this with their physician.
NeuroStar TMS Therapy should not be used in patients with implants controlled by physiological signals. This includes pacemakers, implantable cardioverter defibrillators (ICDs) and vagus nerve stimulators (VNS).
Contact our office in Katy today to schedule a consultation with Dr. Guerrero to see if you are a candidate for this exciting new therapy.




